AK-FLUOR®
Fluorescein Injection, USP
LONG GROVE LABEL







LGP-215
LONG GROVE LABEL







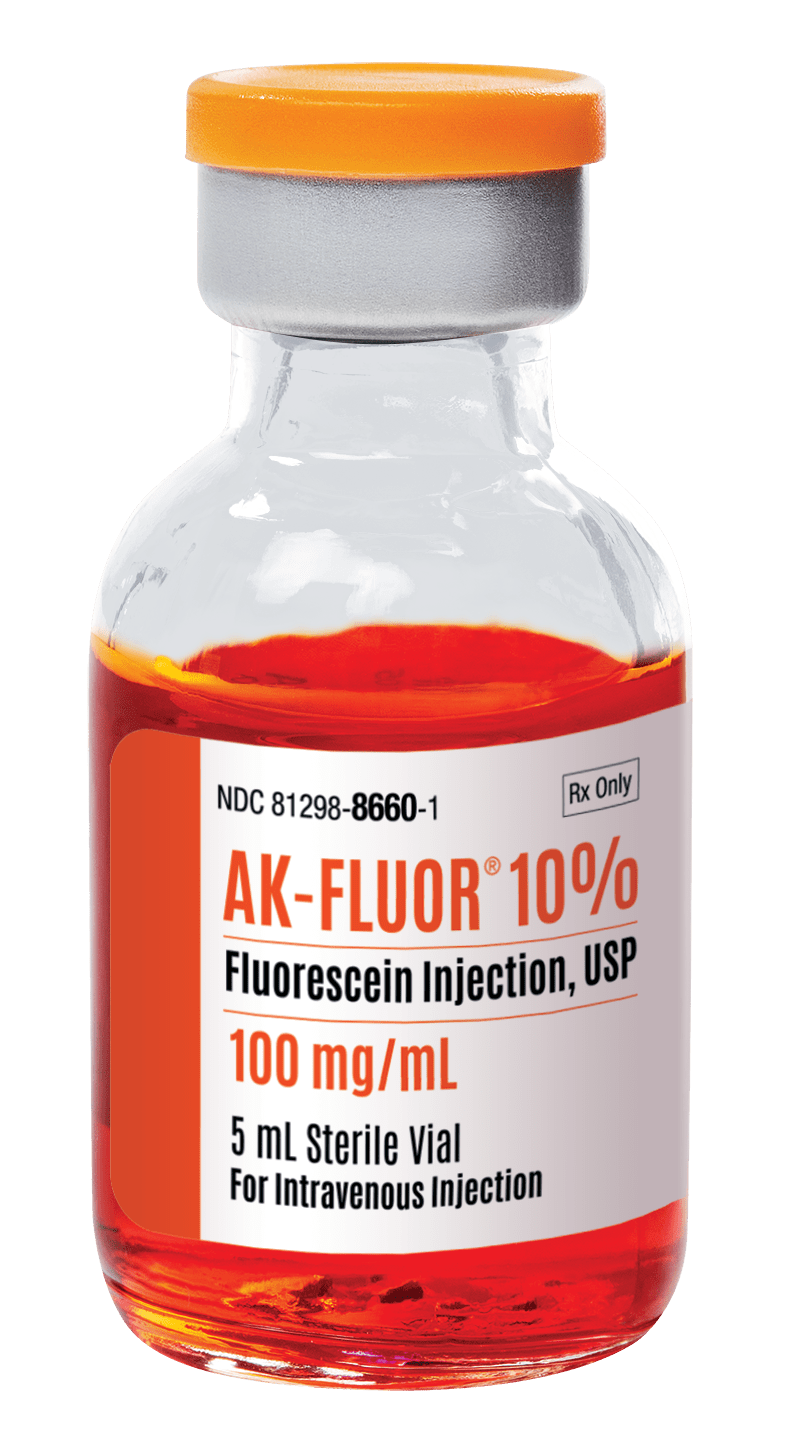
| UNIT OF SALE NDC |
|---|
| 81298-8660-3 |
| CONCENTRATION |
| 100 mg/mL |
| FILL VOLUME |
| 5 mL |
| UNIT OF SALE |
| 12 |
| BESSE | CARDINAL | Cencora |
|---|---|---|
| 10293492 | 5948658 | 10293492 |
| McKesson | McKesson Med-Surg | Morris & Dickson |
| 2991610 | 1260615 | 428854 |
 |
 |
| UNIT OF USE NDC: | UNIT OF SALE NDC: |
| 81298-8660-1 | 81298-8660-3 |
| VIAL SIZE: | 5 mL |
| VIAL CLOSURE: | 13 mm |
| PACK SIZE: | 12 Vials |
| CASE SIZE: | 24 Cartons of 12 Vials |

| UNIT OF SALE NDC | CONCENTRATION | FILL VOLUME | UNIT OF SALE |
|---|---|---|---|
| 81298-8660-3 | 100 mg/mL | 5 mL | 12 |
| BESSE | CARDINAL | CENCORA |
|---|---|---|
| 10293492 | 5948658 | 10293492 |
| MCKESSON | MCKESSON MEDICAL-SURGICAL | MORRIS & DICKSON |
| 2991610 | 1260615 | 428854 |
 |
 |
| UNIT OF USE NDC: | UNIT OF SALE NDC: |
| 81298-8660-1 | 81298-8660-3 |
| VIAL SIZE: | 5 mL |
| VIAL CLOSURE: | 13 mm |
| PACK SIZE: | 12 Vials |
| CASE SIZE: | 24 Cartons of 12 Vials |
LGP-215
To order Long Grove products, please contact your wholesaler or contact customer service.
To order Long Grove products, please contact your wholesaler or contact customer service.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |